WARNING of NEUROLOGICAL REACTION must accompany new RSV TOXIC JAB after string of cases involving Guillain-Barré syndrome
07/09/2025 / By S.D. Wells
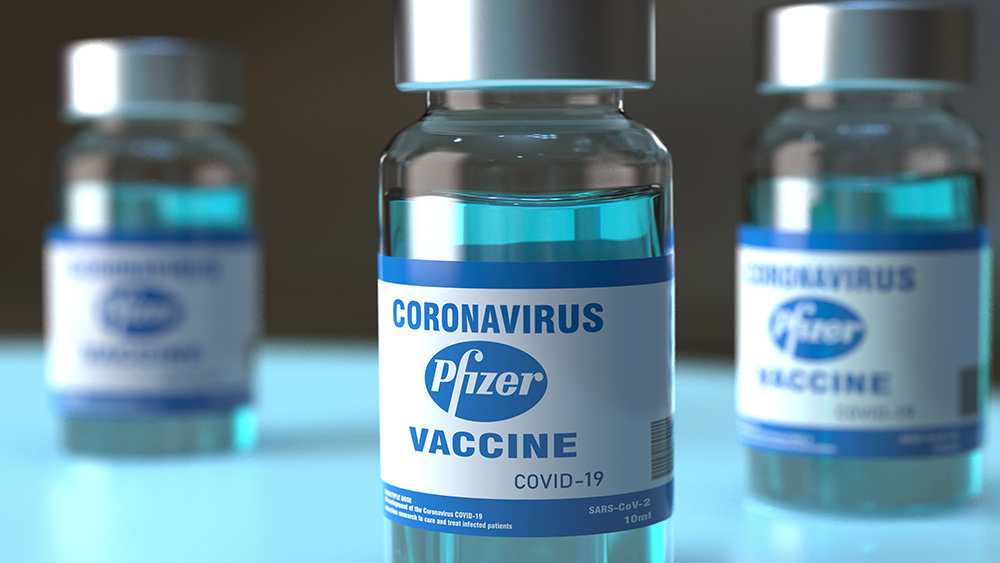
Vaccines are no longer safe for humans. They may have been at some point in history, but now the neurotoxins and spike prions are just too much for the central nervous system to handle, and people are suffering dire consequences from the lethal injections, including the clot shots by Pfizer, and namely the RSV jab.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has issued an alert after rare neurological complications were linked to two respiratory syncytial virus (RSV) vaccines—Abrysvo, developed by Pfizer, and Arexvy, by GSK. While the vaccines remain approved for use and widely recommended, healthcare professionals are now required to warn patients of the small but serious risk of developing Guillain-Barré syndrome (GBS), a rare neurological condition that requires urgent hospital care.
- UK health regulator MHRA has issued a warning for two RSV vaccines—Pfizer’s Abrysvo and GSK’s Arexvy—after 21 cases of Guillain-Barré syndrome (GBS), a rare but serious neurological condition, were reported among older adults who received Abrysvo.
- Healthcare staff are now required to directly inform patients about the potential risk of GBS, although the vaccines are still recommended due to the high risk RSV poses—particularly to elderly and pregnant individuals.
- Guillain-Barré syndrome affects the nervous system, causing symptoms like tingling, muscle weakness, and in severe cases, difficulty breathing or swallowing; while most recover, some may suffer lasting nerve damage.
- Out of nearly 2 million Abrysvo doses administered, only 21 GBS cases have been reported, and none with Arexvy so far in the UK; experts stress the link is not proven, and such rare reactions may occur coincidentally or as immune system misfires.
Health Regulator Issues Warning as Vaccine Given to Millions Is Linked to Debilitating Illness
The warning follows 21 reports of suspected GBS in patients over the age of 60 who received Abrysvo. All reported cases involved individuals aged 75–79. The alert emphasizes that these incidents occurred out of nearly 2 million doses administered in the UK, indicating that the reaction remains very rare—estimated at about 1 in 1,000. To date, no GBS reports have been linked to Arexvy, though its use has been far more limited in the UK.
Guillain-Barré syndrome is an autoimmune condition in which the body’s immune system mistakenly attacks nerve cells. It typically begins with tingling and weakness in the limbs and may progress to affect breathing, swallowing, and even heart function. While most people recover within a year, some suffer permanent nerve damage.
The MHRA’s alert requires NHS staff to provide direct verbal warnings to patients—particularly older adults and pregnant women—who are being offered either vaccine. Previously, such risks were only detailed in patient safety leaflets. The MHRA also urged anyone experiencing symptoms of GBS after vaccination, such as numbness, muscle weakness, or difficulty moving or breathing, to seek immediate medical attention.
Despite the new warning, the MHRA, like its US counterpart the FDA, maintains that the benefits of RSV vaccination outweigh the risks. RSV is a highly contagious virus that spreads through droplets, close contact, or contaminated surfaces. It causes severe respiratory illness, especially in infants and the elderly, leading to approximately 8,000 adult and 100 infant deaths annually in the UK, along with many hospitalizations.
RSV vaccines are a recent addition to public health tools in the UK, only approved in the past year. Abrysvo is provided through the NHS, while Arexvy is available privately. Pregnant women are offered the RSV jab to pass immunity to their unborn babies, with studies showing a 70% reduction in severe infection risk for newborns. Current NHS data shows 62% of eligible seniors and 50% of pregnant women in England have received the vaccine.
The Yellow Card scheme, used to monitor adverse drug reactions in the UK, flagged the potential GBS cases. While this system helps identify possible safety issues, it does not prove causality. Many GBS cases may be coincidental or triggered by infections rather than the vaccine itself.
Nevertheless, the alert underscores the importance of monitoring, transparency, and informed consent as new vaccines continue to roll out, particularly for vulnerable populations. Bookmark Plague.info to your favorite independent websites for updates on deadly vaccines and the TWO HUNDRED new gain-of-function viruses NIH, CDC and WHO plan to release into the “wild” while blaming infected bat soup eaters at the Wuhan wet market.
Sources for this article include:
Submit a correction >>
Tagged Under:
dirty vaccine vaccine damage, Guillain-Barre, medical violence, rsv, RSV vaccine, vaccine injury, vaccine nightmare, vaccine wars
This article may contain statements that reflect the opinion of the author